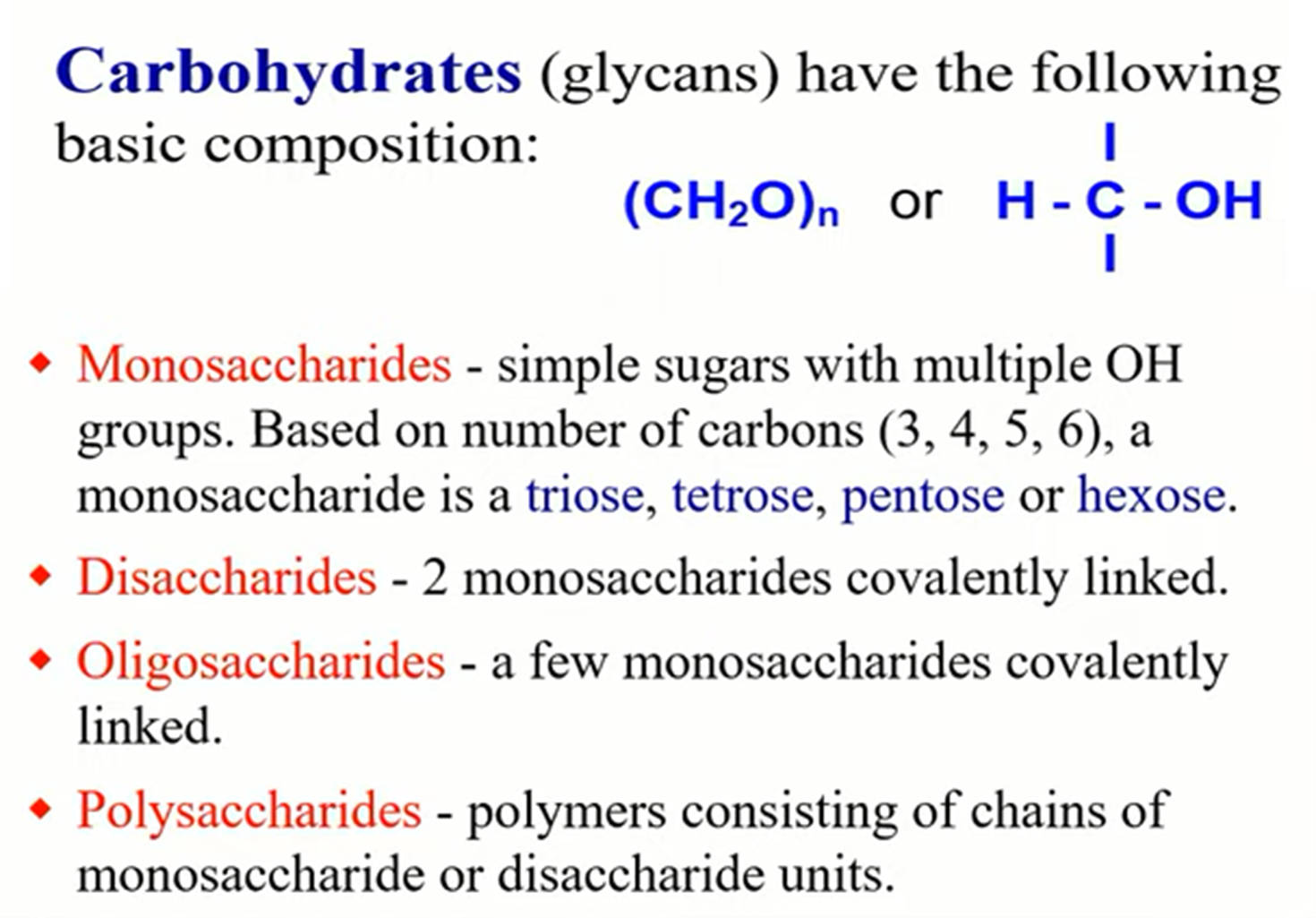
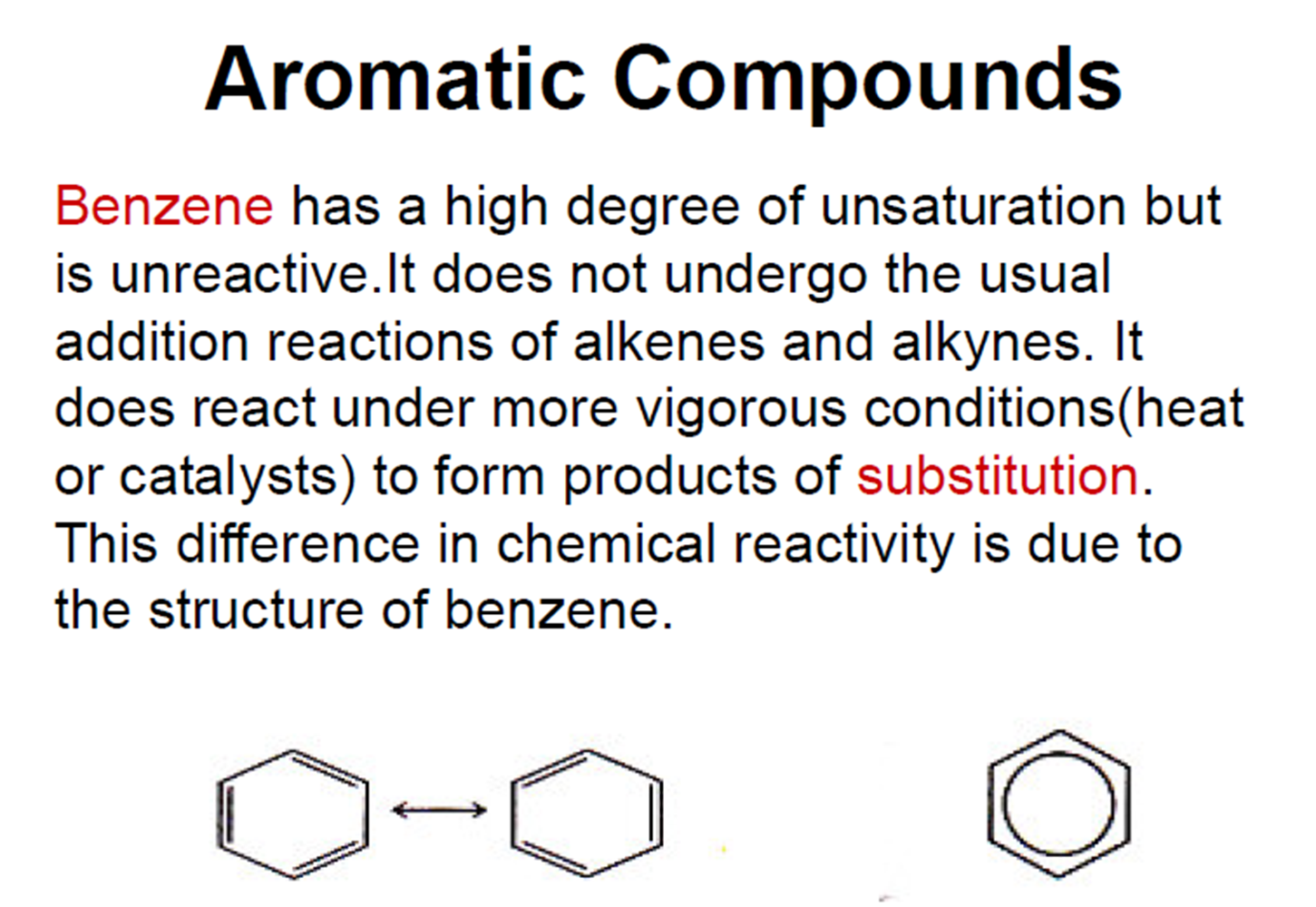
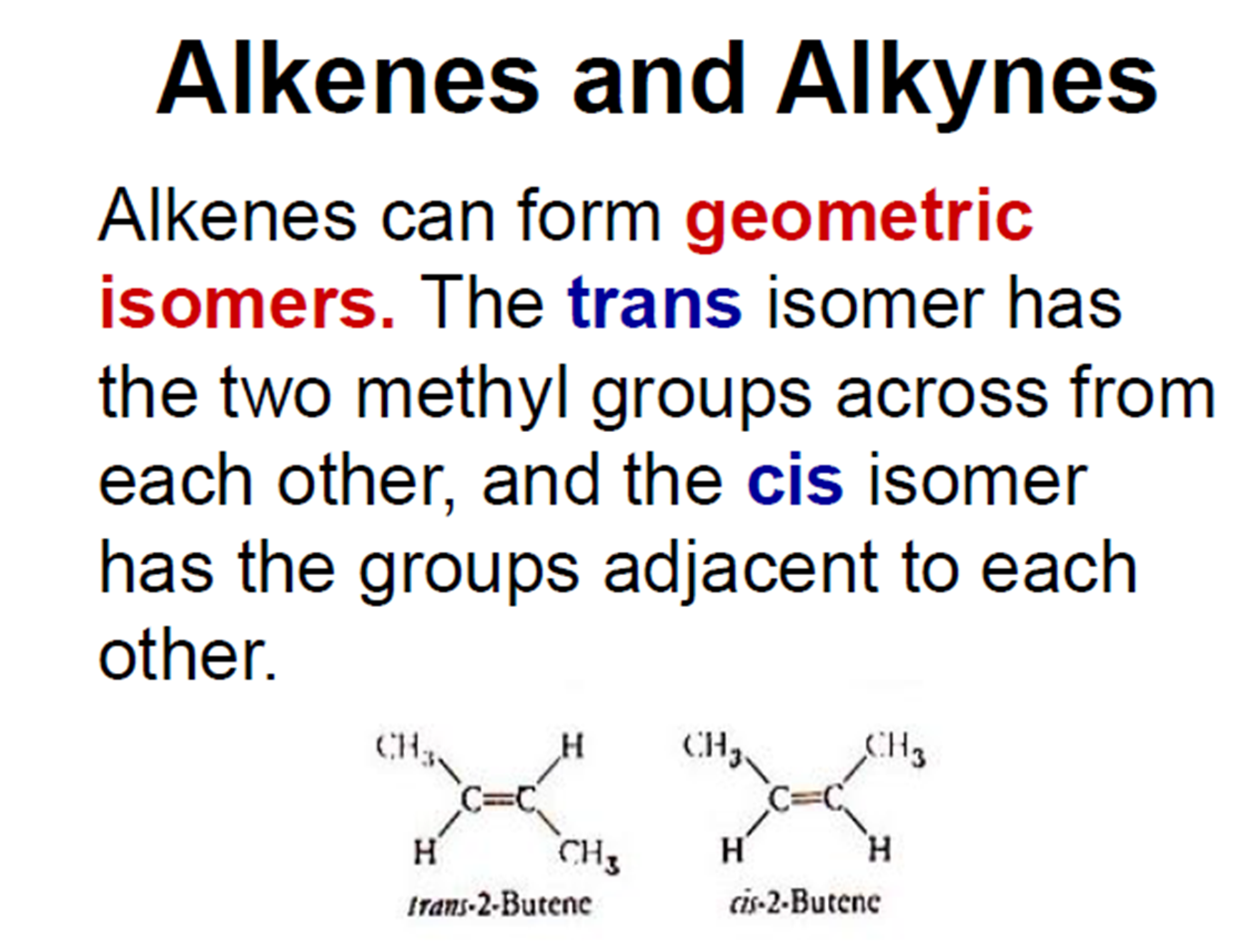
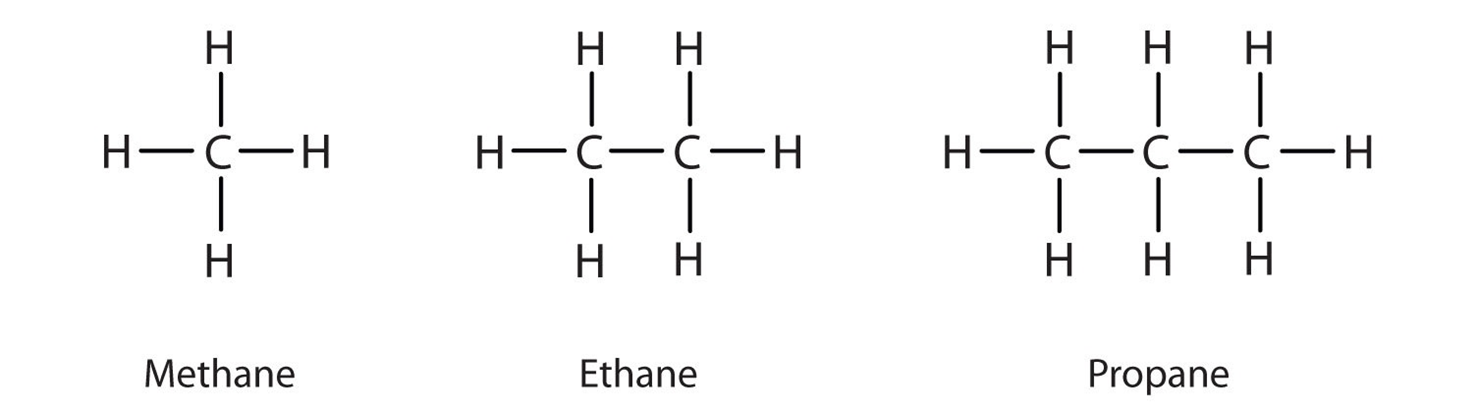
Chimical proprites of acids and bases
In 1923, the Danish chemist Bronsted and the English chemist Lowry independently presented their theories of acids and bases on the basis of proton-transfer. According to this concept:
An acid is a substance (molecule or ion) that can donate a proton (H+) to another substance.
A base is a substance that can accept a proton (H+)from another substance.
For example, HCl acts as an acid while NH3 acts as a base.
pollution
pollution is the introduction of contaminates into a natural environment that causes instability, disorder, harm or discomfort to the Ecosystem i.e. physical system or living organisms.
pollution can take the form of chemical substances or energy, such as noise, heat, or light.
Acids, Bases and Salts
Acids and Bases are substances with specific Physical and chemical properties. we can determine if substances acidic or basic by testing their pH or by indicators.
solubility and solution colloidal system
Acid-base balance, buffer systems and pH blood
Cellular mechanisms of regulation of acid-base
cellular mechanisms of maintaining constancy of hydrogen ions concentration in extra-cellular fluid: